
The first volume of Frank Herbert’s Dune series debuted back in 1965. Mining a precious natural substance called spice melange was a driving theme in that epic space saga. This spice granted people the ability to navigate vast expanses of the cosmos. It also became the basis of an intergalactic civilization. That was, of course, fiction.
Back here on Earth, in real life, a group of metallic elements has made possible our own technology-driven society. Called rare earths, these 17 elements are crucial to nearly all modern electronics. And demand for these metals has been skyrocketing.
Fifteen rare earths make up a whole row on most periodic tables. Known as lanthanides, they run from lanthanum to lutetium — atomic numbers 57 through 71. Also included in the rare earths are scandium (atomic number 21) and yttrium (atomic number 39). Those last two elements tend to occur in the same ore deposits as lanthanides. They also have similar chemical properties.
The rare earth cerium can serve as a catalyst to process crude oil into a host of useful products. Nuclear reactors rely on another: gadolinium. It captures neutrons to control the production of energy by a reactor’s fuel.
Rare-earth mining is dirty but key to a climate-friendlier future
But the most outstanding capabilities of rare earths are their luminescence and magnetism. For instance, we rely on rare earths to color our smartphone screens. They fluoresce to signal that euro banknotes are the real deal. They relay signals through fiber-optic cables along the seafloor. They also help build some of the world’s strongest, most reliable magnets. These metals generate sound waves in your headphones and boost digital data through space.
More recently, rare earths have been driving the growth of green technologies, such as wind power and electric vehicles. They may even give rise to new parts used in quantum computers.
“They’re everywhere,” says Stephen Boyd of these metals. He’s a synthetic chemist and independent consultant based in Dixon, Calif. When it comes to the uses of rare earths, he says, “The list just goes on and on.”
Superpowers trace to their electrons
Rare earths tend to be malleable (easy to deform). These metals also have high melting and boiling points. But their secret power lies in their electrons.
All atoms have a nucleus surrounded by electrons. Those tiny electrons inhabit zones called orbitals. Electrons in the orbitals farthest from the nucleus are known as valence electrons. They take part in chemical reactions and form bonds that link atoms together.
Explainer: What is a metal?
Most lanthanides possess another important set of electrons. These “f-electrons” dwell in a Goldilocks zone. It’s located near the valence electrons but slightly closer to the nucleus. “It’s these f-electrons that are responsible for both the magnetic and luminescent properties of the rare-earth elements,” says Ana de Bettencourt-Dias. She’s an inorganic chemist at the University of Nevada, Reno.
When stimulated, rare-earth metals radiate light. The trick is to tickle their f-electrons, says de Bettencourt-Dias. An energy source such as a laser beam can jolt one f-electron in a rare earth element. The energy boosts the electron into an excited state. Later, it will drop back to its starting — or ground — state. As they do, these f-electrons emit light.
Place on the periodic table
The group of 17 elements (highlighted in blue on this periodic table) are known as rare earths. A subset of them, known as the lanthanides — lutetium, Lu, plus the row starting with lanthanum, La — appear in a single row. Rare-earth elements have a subshell of electrons (called f-electrons) that give these metals magnetic and luminescent properties.
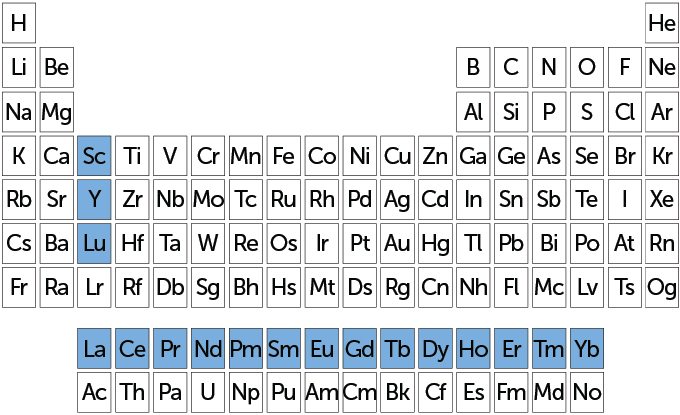
The rare-earth glow
After being excited, each rare earth reliably emits precise wavelengths (colors) of light, de Bettencourt-Dias notes. This allows engineers to carefully tune the electromagnetic radiation (light) in many electronics. Terbium, for instance, emits light at a wavelength of about 545 nanometers. That makes it good for creating green-glowing phosphors in the screens used in TVs, computers and smartphones. Europium, which has two common forms, is used to make red and blue phosphors. Such phosphors can paint screens with most shades of the rainbow.
Rare earths also radiate useful invisible light. Yttrium is a key ingredient in yttrium-aluminum-garnet, or YAG, crystals. They form the core of many high-powered lasers. Engineers tune the wavelengths of these lasers by lacing YAG crystals with another rare earth. The most popular: a neodymium-laced YAG laser. These are used for a broad range of things — from slicing steel and removing tattoos to laser range-finding. And erbium-YAG laser beams are a good option for certain surgeries. They won’t slice too deeply because their light is readily absorbed by the water in our tissues.
Beyond lasers, lanthanum is crucial for making the infrared-absorbing glass in night-vision goggles. “And erbium drives our internet,” says Tian Zhong. He’s a molecular engineer at the University of Chicago in Illinois. Much of our digital data travels through optical fibers as light. It typically has a wavelength of about 1,550 nanometers — the same as erbium emits. The signals in fiber-optic cables dim as they travel far from their source. Because those cables can stretch for thousands of kilometers across the seafloor, erbium is added to fibers to boost their signals.

The source of mighty magnets
In 1945, scientists constructed the world’s first programmable, general-purpose digital computer. Its formal name was ENIAC. But scientists quickly nicknamed it the “Giant Brain.” And that was apt. It weighed more than four elephants and covered an area roughly two-thirds the size of a tennis court.
Less than 80 years later, our smartphones boast far more computing power than ENIAC ever had. Society owes this shrinking of electronic technology in large part to the exceptional magnetic power of rare earths. And those f-electrons are the reason why.
Rare earths have many orbitals of electrons, but the f-electrons inhabit a specific group — or subshell — of seven orbitals. Each orbital can house up to two electrons. But most rare earths contain multiple orbitals in this subshell with just one electron.
Neodymium atoms, for instance, possess four of these loners. Dysprosium and samarium are two rare earths with five loner electrons. Crucially, those unpaired electrons tend to point — or spin — in the same direction, Boyd says. “That’s what creates the north and the south poles that we classically understand as magnetism.”

These lone f-electrons flitter behind a shell of valence electrons. That somewhat shields their synchronized spins from heat and other demagnetizing forces. And that makes these metals great for building permanent magnets, Zhong says.
The magnetic fields in permanent magnets, like the ones that hold up pictures on a fridge door, arise from the magnets’ atomic structure. (Electromagnets, in contrast, need an electric current. Turn it off and the magnetism turns off, too.)
But even with their shielding, rare-earth magnets have limits. Pure neodymium, for example, readily corrodes and fractures. Its magnetic pull also starts to lose strength above 80° Celsius (176° Fahrenheit). So manufacturers often make alloys of rare earths with some other metals. This makes those magnets more resilient than if they had been made from rare earths alone, says Durga Paudyal. He’s a theoretical physicist at Ames National Laboratory in Iowa.
This alloy approach works well, he adds, because some rare earths can orchestrate the magnetic fields of other metals. Just as weighted dice will preferentially land on one side, some rare earths — such as neodymium and samarium — exhibit stronger magnetism in certain directions. It’s because the orbitals in their 4f-subshells are unevenly filled. This directionality can be used to coordinate the fields in other metals, such as iron or cobalt. The result: robust, extremely powerful magnets.
The role for these super magnets
The most powerful alloy magnets are NIBs — a mix of neodymium, iron and boron. A 3-kilogram (6.6-pound) NIB magnet can lift objects more than 100 times its weight. More than 95 percent of the world’s permanent magnets are made from this rare-earth alloy. These are the magnets that generate vibrations in smartphones and produce sounds in earbuds and headphones. They enable the reading and writing of data on hard-disk drives. They also create the magnetic fields used in MRI machines.
Adding a bit of dysprosium to these magnets can boost their heat resistance. Now they become a good choice for the rotors that spin in the hot interiors of the motors driving many electric vehicles.
Developed in the 1960s, a samarium-cobalt alloy went into the first popular rare-earth magnets. Though slightly weaker than NIB magnets, samarium-cobalt ones have superior resistance to heat and corrosion. That makes them great for use in high-speed motors, generators, speed sensors in cars and airplanes — and in the moving parts of some heat-seeking missiles. Samarium-cobalt magnets also form the heart of the devices used to boost signals emitted by most radar systems and communications satellites. Some of these rare-earth-based signal boosters are transmitting data from the Voyager 1 spacecraft. Launched in September 1977, that craft is the most distant human-made object — already more than 23 billion kilometers (14 billion miles) away.
Recycling rare-earth elements is hard — but worth it
Strong and reliable, rare-earth magnets are at the heart of many green technologies, too. They’re in the motors, drivetrains, power steering and many other parts used in electric cars. Tesla’s use of neodymium-alloy magnets in its farthest-ranging Model 3 cars has sparked worries that magnet-makers may soon find it hard to get enough neodymium (which is mined largely in China).
Rare-earth magnets also replace gearboxes in many offshore wind turbines. They help boost the turbines’ efficiency and cut their need for servicing. And in August, Chinese engineers introduced “Rainbow.” It’s the world’s first magnetically levitated train line to rely on rare earths. Its magnets enable the trains to float above their tracks without consuming electricity.
Rare earths may even soon advance quantum computing. Conventional computers store and record data as binary bits — 0s and 1s. Quantum computers instead use quantum bits. Also called qubits, they can occupy two data states at once. Crystals containing rare earths make good qubits, Zhong says, because their shielded f-electrons can store quantum data for long periods of time. One day, scientists might even manipulate the light-emitting properties of rare-earth qubits to share information between quantum computers. It could give birth to a quantum internet, Zhong says.
It’s too early to predict exactly how rare-earth metals will boost the expansion of all these emerging technologies. But it’s probably safe to say: Rare earths better not be too rare, because we’re going to need a lot of them.
SOURCE: SCIENCE NEWS EXPLORES
