Imagine if you cut yourself and saw purple blood gush out of your skin. Now that’ll be the start of an alien invasion or another sign of the end times. If you’re lucky to have the experience indoors, you’ll save yourself the ‘witch hunt’ from the public. However, this may not be as strange as you think, because blood is not always red.
Don’t be alarmed if you come across green, blue and even purple blood.
The colour of blood is to do with the compound that transports oxygen around the body. For most mammals and vertebrates this is haemoglobin. Haemoglobin is a protein made up of four chains known as globins and at the centre of each globin is a molecule called haem which contains iron.
In simple terms:
(2 alpha globins + 2 beta globins) + Haem/Iron = Haemoglobin
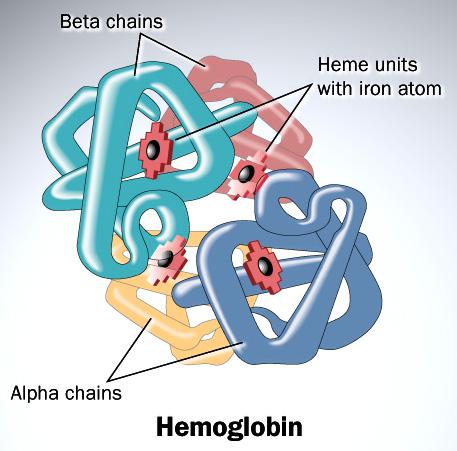
It’s the iron at the centre of the globin chains that give blood its red colour and the intensity of the redness varies based on the interaction with oxygen. As the heart pumps blood around the body, its constituents change. Haemoglobin carries Oxygen from the lungs to the tissues through the arteries and transports Carbon dioxide back from the tissues to the lungs through the veins. The reaction of Oxygen with iron causes the redness. Oxygenated blood in the artery is therefore bright red whereas deoxygenated blood in the vein is dark red. In the absence of oxygen, blood is not always red.
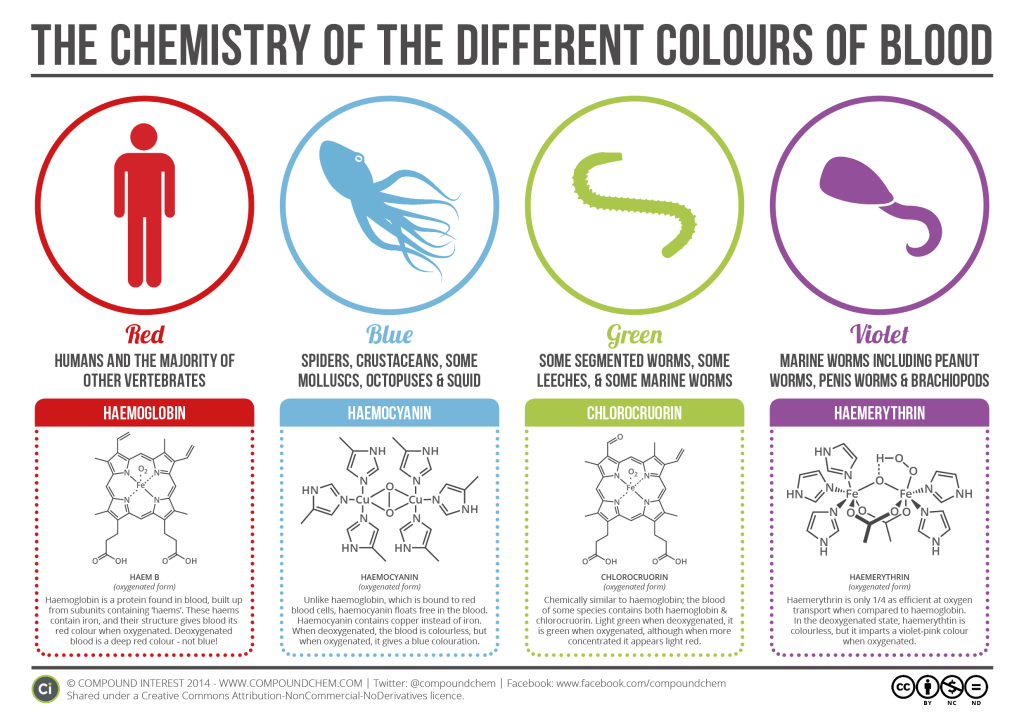
Why blood is not always red
When the iron at the centre of the haemoglobin molecule is replaced by a different molecule or interactions with the haemoglobin and certain drugs causes a change in the normal form of the iron molecule, this can cause a change in the colour of blood from red to another colour.
For example, a drug called sumatriptan which is used as a treatment for migraine contains sulphur which causes the blood of some people on the medication to go green due to the interaction of the sulphur with the iron. The condition is known as Sulfhemoglobinemia and although rare has been reported in some individuals.
In most species of octopus, some crabs and other crustaceans, the blood carrying molecule is haemocyanin with a copper rich centre and the interaction of the copper with oxygen gives their blood a blue colour. In the absence of oxygen the blood is colourless.
Lobsters, earth worms and leeches have green blood. Their oxygen carrying molecule is known as chlorocruorin. Also with an iron centre, the interaction with oxygen gives the blood a green colour which turns light green when deoxygenated.
Some worms have another type of haemoglobin known as haemerythrin. Also with an iron centre, the difference in structure means the interaction with oxygen makes their blood purple and in the absence of oxygen this turns colourless.
As you can see, blood is not always red and the infographic below nicely summarises the chemistry behind the colour of blood. (Thanks to CompoundChem for the infographic)