From the Ten Most Beautiful Experiments by George Johnson
In 1896, Robert Millikan, a young physicist fresh out of Columbia University with a PhD, found himself at a lecture in Berlin where Wilhelm Roentgen was showing pictures he had taken of the bones inside a hand. Two years earlier Robert Millikan had heard the great Albert Michelson speculate that physics was all but over. The laws of motion and optics were set firmly in place and Maxwell’s equations had drawn tight the threads Faraday and his generation had spun between electricity and Magnetism.
Heinrich Herts had gone on to verify Maxwell’s theory, showing that radio waves can be reflected, refracted, focused, and polarized. But there was a new entirely unexpected phenomenon known as the X-rays. Roentgen made an astounding discovery while investigating the glowing spot that appears at the end of an evacuated glass “discharging tube” when a large enough current is applied across two metal plates inside a negatively charged cathode and positively charged anode. Travelling through the rarefied air these cathode rays were puzzling enough.
Robert Millikan Watching The Start of X-Rays
When William Crookes used a Maltese cross in a tube designed with an obstruction inside its shadow appeared on a fluorescing glass, a clue that the rays moved bulletlike in straight lines. If he held a magnet near the tube the beam would sway to one side. Mount a gemstone inside and it would fluoresce. The rays seemed to have substance turning the vanes of a tiny paddle wheel. “A fourth state of matter” Crookes claimed-solid, liquid, and gaseous and radiant. Roentgen found that if the beam struck the end of the tube with enough force, it unleashed a different kind of radiation-powerful enough to penetrate flesh. Less than a year later Henri Becquerel in Paris discovered another form of penetrating rays emanating from lumps of uranium, passing through an opaque shield and leaving their mark on a photographic plate. Both kinds of radiation, it soon was learned could ionize a gas, giving it an electrical charge. We know that they do this by knocking electrons off atoms.

Robert Millikan returned from Europe to take a job at the University of Chicago. Millikan watched from afar as some of the greatest scientists explored the new physics. J.J Thomson showed that beams could be repelled not just by magnets but by strong electrical fields. Hertz tried and failed at this experiment and Thomson suspected that Hertz hadn’t pumped enough air from the tube which meant the lingering molecules were shorting out the plates as surely as if they had been rained on. With a better vacuum, he was able to nudge the beam toward the positive pole which was a strong indication that cathode rays were made of negatively charged matter, particles of electricity (electrons).
It was in 1906, Robert Milikan felt disappointed in himself that at the age of 38 he had made no important discoveries. He knew Thomson’s experiment was impressive but hadn’t clinched the case. For all anyone knew, electrons came in a slew of charges and sizes all yielding the same ratio. Thomson had just assumed they were identical. The Germans remained particularly skeptical in the face of this uncertainty that electricity was an aethereal wave.
Robert Millikan began by repeating an experiment in which a scientist in Thomson’s lab had timed how quickly a charged mist of water vapor, one that had been ionized with X-rays or radium settled to the bottom of a closed container. Above and below the cloud were metal plates connected to the poles of a battery. By observing the descent, you could calculate its total charge. Divide that by your guesstimate of how many charged particles were in the cloud and you could rough out an average value for the electron. The technique which involved a device called a Wilson cloud chamber, was rife with uncertainty and assumptions. The vapor was continually evaporating, leaving the top edge of the cloud so irregular and indistinct that tracking its motion was an exercise in frustration. Millikan cranked up the voltage, hoping he could hold the target steady suspended between positive and negative. Then he could measure the rate of evaporation and account for it in his calculation. Instead, he flicked on the switch and blew the cloud away.
The experiment seemed like a failure until he noticed that a few individual water drops remained hanging in the air, just the right weight and charge so that the downward pull of gravity was offset by the levitating power of the electrical field. Realizing this would make a more decisive experiment he observed them one by one . Peering through a small telescope set up two feet away, he would pick a drop hovering in suspension and then suddenly turn off the voltage. He recorded the data, comparing the estimate of the drop with how much charge was required to keep it afloat.
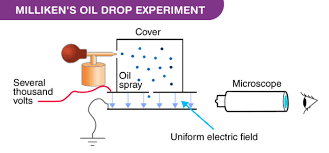
In September 1909, Robert Millikan travelled to Winnipeg to present his results to the British Association of the Advancement of Science. Rutherford lectured on the state of atomic physics noted that for all the recent successes it had not yet been possible to detect a single electron. Then Millikan who was not even on the agenda surprised everyone by reporting that he had come close to doing that. After arriving in Chicago, he asked Harvey Fletcher to see if the droplet experiment could be done with something less evanescent than drops of water. Purchasing a perfume atomizer and watch oil, Fletcher began assembling the equipment . After assembling he sprayed a mist of oil above the apparatus and watched through a telescope. “I saw a most beautiful sight.”
By the next morning Fletcher had wheeled in a large bank of batteries capable of producing a thousand volts and connected them to brass plates. Turning on the current he watched with excitement as some of the droplets were pushed slowly upward while others were pulled down, the friction from the tiny nozzle of the atomizer having given them negative or positive charges. When Millikan saw how well the plan was working, he was elated. He and Fletcher refined the setup and spent nearly every afternoon for the next six months taking data.
Millikan’s data on the water drops had already come under attack from an Austrian experimenter who soon was claiming to have found sub electrons and suspected that there was no smallest unit of charge. But Robert Millikan had found with his earlier, cruder experiment was confirmed in spades by the oil drops. There really were electrons. One afternoon, Charles Proteus Steinmetz, the pioneering electrical engineer came to watch the experiments. “I never would have believed it,” he said shaking Fletcher’s hand. “I never would have believed it.”
Early 1910 they began writing up the results and over the next three years Millikan continued to improve the experiments. The simple tabletop contraption morphed into a high-tech device with filtered air, tightly regulated temperature, pressure and voltage and a clock capable of marking time in milliseconds. “He who has seen that experiment has in effect seen the electron” Millikan later wrote. “He can count the number of electrons in a given small electrical charge with exactly as much certainty as he can attain in counting his fingers and his toes.
The story had a strange denouement after Millikan’s former assistant Harvey Fletcher died in 1918. A memoir surfaced describing both his appreciation to Millikan for advancing his career and his disappointment for not getting more recognition for the oil drop experiment. Fletcher’s insistence that his account be published posthumously added to its credibility but also Millikan opportunity to respond.
Robert Millikan’s Legacy
Though Robert Millikan was the indisputable force behind the isolation and measurement of the electron, he probably could have been more generous to his student. The beauty here lies with the experiment not the experimenter. Millikan was later accused of cooking books. More interesting than the unfounded allegations is the question of how you keep from confusing your instincts with your suppositions unconsciously nudging the apparatus. It is something every experimenter must struggle with. The most temperamental piece of laboratory equipment will always be the human brain.
